Super Bubble Maker

Have science fun as a family! Complete activities with parental supervision.
Materials:
- Small flat pan or container (Example: Plastic shoebox, baking pan, etc.)
- 2 cups warm water
- 1/3 cup dish soap
- 3 tablespoons white sugar
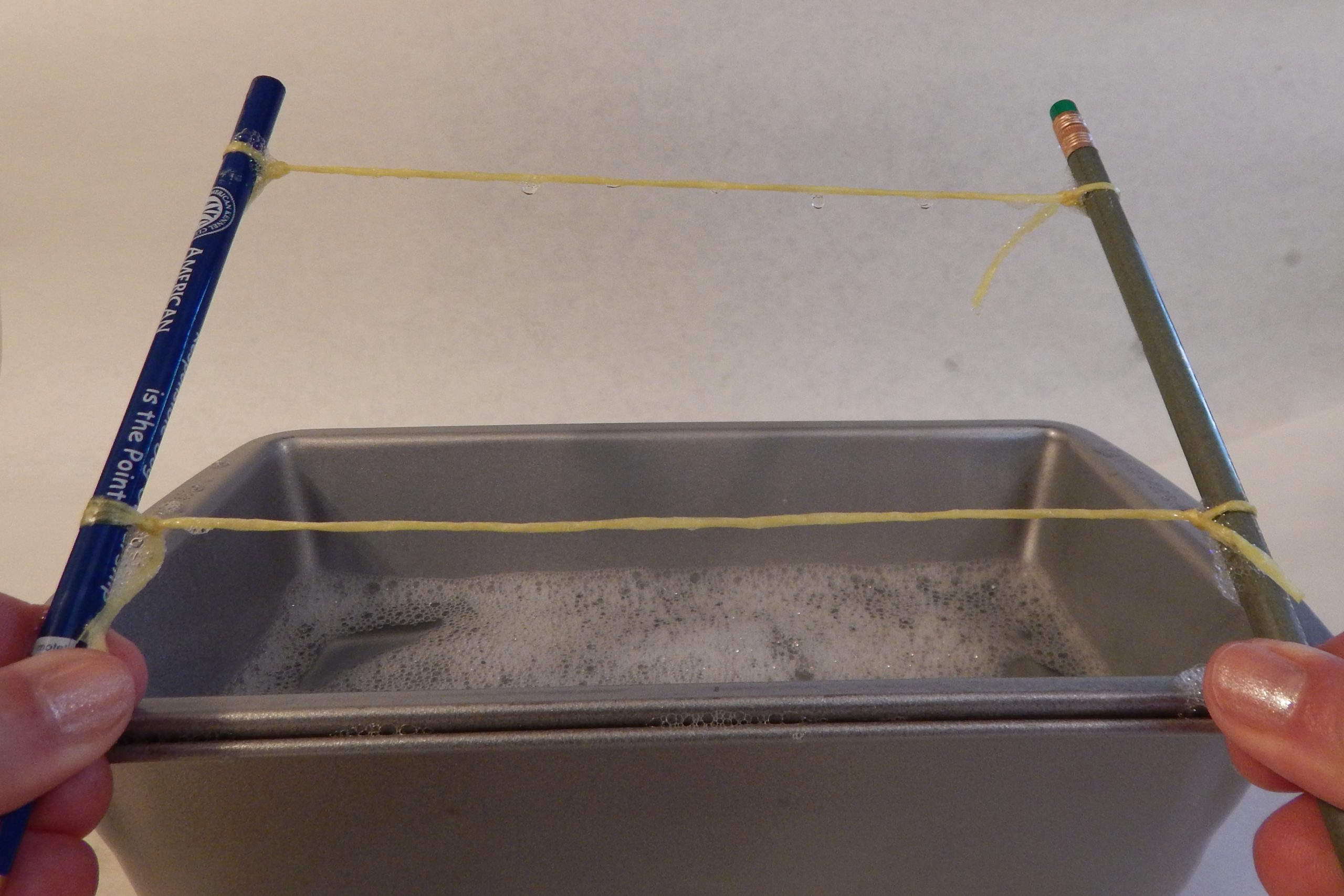
- 2 pencils or sticks
- Approximately two feet of string
- Scissors
Procedure
- Mix the white sugar into the warm water until it dissolves. Adding sugar makes the water in the bubble evaporate more slowly, so your bubbles will last longer!
- Then slowly stir in the dish soap. Try not to stir too quickly, as frothy, sudsy water is not ideal for bubble making.
- Let this solution rest, undisturbed for at least one hour or more. Optional: place in the refrigerator for more durable bubbles.
- In the meantime, tie two pencils together with two pieces of string to form a rectangle. Make sure the knots are tight and cut off any extra string.
- Once your solution has rested, take it outside begin your bubble experiment!
- To make a super bubble, make sure the two pencils and all the string are dipped into the bubble solution. With a pencil in each hand, slowly pull it out and stretch the string straight to form a rectangle. See the soapy film stretched across? If not, try dipping again.
- Now slowly move the entire rectangle. The air will push against the soapy film. It will either stretch and form a bubble sphere, or stretch and burst. It takes practice!
- Your bubble maker is a rectangle, but what shape is the bubble? Why are bubbles typically a sphere shape?
- Be sure to take a picture or video to share in the Facebook comments on the Buffalo Museum of Science or Tifft Nature Preserve pages!
What’s it all about?
Water molecules like to stay together. You can observe this in the formation of water droplets. The water molecules making up the droplets are more attracted to each other than the air around them.
This is called surface tension. By adding soap to the water, you are decreasing the surface tension or ability of the water molecules to bond tightly together. This makes the surface more flexible for bubble making.
Bubbles are usually sphere-shaped because the existing surface tension is always striving to shrink the bubble into a shape with the smallest surface area, and that shape is a sphere (compared to other shapes like a cube.)
Try It!
- Try experimenting with different ingredients and different shaped homemade bubble wands. How can you make the biggest bubble, the longest lasting bubble, or the prettiest bubble?
- You can create a wide variety of bubble wands and blowers from everyday materials. Tie a string or a chenille stem into a loop. Use your imagination and let us know what worked for you!